Crystallization
The process by which broken materials that exhibit crystalline lattices are slowly rebuilt to create crystals of controlled size and purity.
View our Products
NRV Series
An oil-sealed rotary vane vacuum pump is often used for vacuum pressure swing adsorption applications because it is inexpensive, efficient, and long-lasting. The rotary vane is cheaper in both installation and operation over time compared to dry screw, liquid ring, and dry claw vacuum pumps. This is due to its compact size and simple design with one moving part. This translates to easier installation, simpler maintenance, and faster repairs. The circular design of the rotary vane allows for continuous suction with minimal disturbances in the mass transfer rate. The oil seal acts as a lubricant between the vane-to-chamber contact, a seal between the vane and chamber, and a barrier between mildly oxidative or corrosive process gasses and the metal in the pump. These accessories, such as gas ballast kits and inlet filtration kits, make for smoother rotary vane operation for a longer service life. Accessory kits are entirely optional, meaning that you can pick and choose some, all, or none depending on your industry process.
NSP Series
Dry screw vacuum pumps are capable of generating high vacuum levels at medium capacity. This is useful when process requirements are stringent, typically in clean environments for high-quality products.
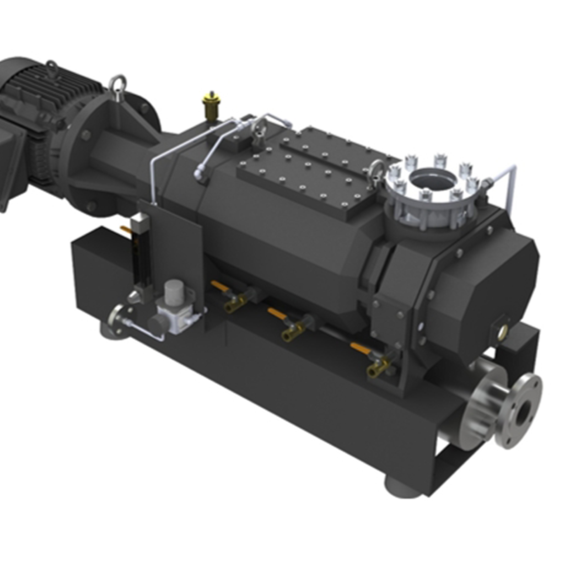
NRB Series
Roots-type blowers are known for their energy efficiency and reliability, as well as their wide range of capacities and capabilities. Depending on the scale of the operation, these units can achieve from double to quadruple-digit capacities, making them versatile for many situations.
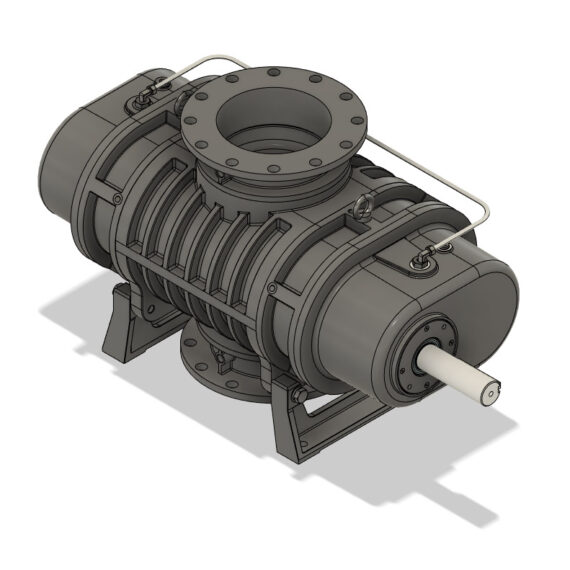
NTC Series
Crystallization involves much moisture; so many dry-running vacuum systems employ a filter or separator element. Moisture will cause uneven heat distribution in dry-running pumps such as screw and lobe pumps, disturbing the thermal expansion of the rotor and potentially causing failure. In oil-lubricated apparatus such as rotary vane vacuum pumps, the moisture mixes and causes the oil to degrade, creating the need for frequent oil changing and increasing operational costs. To simplify the design process, a liquid ring vacuum pump can be used. Condensable process vapors increase the capacity of the liquid ring vacuum pump, which typically lasts for decades with scarce routine maintenance and care.
NTS Series
The NTS effects the same benefits upon a crystallization vacuum system as other liquid ring vacuum pumps. Ranging from 65-4200 CFM with an ultimate vacuum of 28.9”HgV, the NTS Series two-stage port plate liquid ring vacuum pumps are efficient and sturdy, perfect for continuous processes. NES provides a one-year warranty against manufacturing defects.
Crystallization and recrystallization happen inside a crystallizer, a chamber that uses vacuum to manipulate the boiling points of certain liquids. As the pressure in the chamber drops, so too does the temperature at which liquids evaporate.
If we imagine a pot of water on a stove, there would be an invisible column of air pressing down into the pot on the water spanning from the surface of the water up to space. To boil water, the stove keeps adding heat until the boiling temperature, at which point the water has enough energy to break free from the pressure that the air is exerting onto the water. From there, the water evaporates, rising freely until it naturally cools and condenses.
Of course, all liquids have their own boiling points, but not all liquids are okay to boil in this way. Many of the applications of crystallization are used to lower the boiling point for the sole purpose of preserving the compounds within the solution, making sure that the solvent can safely be carried away without burning or degrading the solute within.
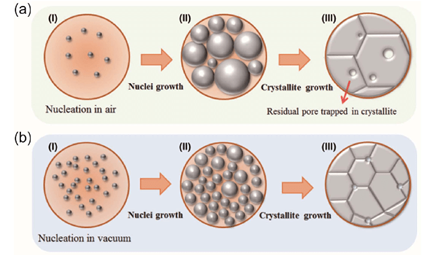
In normal atmospheric pressure and temperature, solvents can only hold so much solute before the solvent cannot dissolve more. For instance, water at room temperature can only hold so much sugar in it before the sugar starts to visibly clump because it will not dissolve. You can adjust this solubility with temperature – hot water will dissolve much more sugar than room temperature water. If you cool the hot water back down to room temperature slowly, the solution will remain in a supersaturated state – it will be holding much more sugar than it usually would at normal temperature.